After reading Pfizer’s March 3, 2023, transcript from their motion to dismiss hearing, I can tell you this brave American hero isn’t going away any time soon.
Mar 18
Brook Jackson is an experienced director of FDA clinical trials who reported dozens of the FDA violations she personally observed while working at Ventavia. Ventavia was one of the clinical trial research organizations contracted by Pfizer to conduct their Phase-3 mRNA vaccine trials.

Ventavia ignored Brook’s reports of the clinical trial violations, so Brook called the FDA directly (a very brave and bold move). Ventavia fired Brook later that same day (shocker).
This post is public so feel free to share it.
Brook was then later contacted by a Pfizer attorney, who mysteriously had her personal cell phone number that her former employee did not even have, in hopes of making her go away.
After reading Pfizer’s March 3, 2023, transcript from their motion to dismiss hearing, I can tell you this brave American hero isn’t going away any time soon.
The Kingston Report is a reader-supported. Consider becoming a free or paid subscriber.
The Honorable Michael Truncale Affirms that Contracts, Facts, and Law are What Matter in Court, Not Subjective Interpretations or Opinions
Under Pfizer’s contract with the US military, Pfizer guaranteed to deliver a safe and effective vaccine in order to get paid.

The vaccine could not be a ‘may be effective vaccine’ or ‘may be safe vaccine’ but must be guaranteed to be safe and effect per their Operation Warp Speed contract.
While EUA (emergency use authorization) laws passed under presidential administrations such as Bush, Jr. and Obama, essentially granted big pharma iron-clad immunity from liability if they manufactured ineffective and unsafe products and then made them available to the American people, Pfizer forfeited this immunity with their safe and effective vaccine guarantee. The safe and effective vaccine guarantee was made more than a dozen times throughout the contract via citations of specific FDA laws and that their vaccines would be safe and effective per conducting standard clinical trials for FDA approved vaccines.

It’s important to note that Phase-3 clinical trials are regulated by laws separate from EUA laws. Pfizer’s attorney tried to persuade Judge Weese that under EUA laws and allegedly under the contract, it is the judge’s responsibility to relieve herself of the case and move it a 3-judge panel (per the law and contract).
Judge Truncale points out to Pfizer’s attorney that the EUA contract clause to move the case to arbitration is precluded by the word ‘may’ and that the word ‘may’ does not mean the same thing as ‘shall.’

Funny how criminals constantly try and change the meaning of words in attempts to get away with their crimes.
Plus Brook Jackson was working on a Phase-3 clinical trial for an FDA-approved product, not an EUA product. (Major, minor detail).
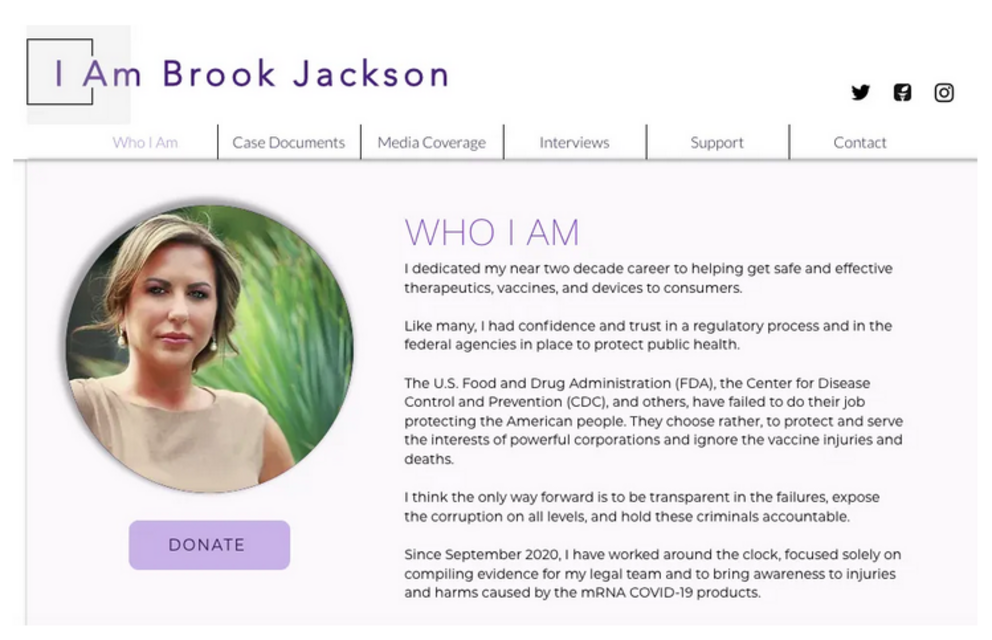
Brook Jackson is a Woman Worthy of Supporting
I have spoken to Brook several times and I consider her a respected colleague, friend, and American hero. She is fighting and winning our battle against Pfizer, and is a real down-to-earth person, who could not remain silent when she witnessed Pfizer’s crimes and knew that innocent children and adults would be harmed if she remained silent.
She has risked and loss her job, and knowingly put her safety at risk by coming forward, even after receiving unsolicited calls from Pfizer’s attorney.
You can support Brook Jackson by donating to her cause here.
The Kingston Report. TRUTH WINS.
Mathew 5:14-16
“You are the light of the world. A town built on a hill cannot be hidden. Neither do people light a lamp and put it under a bowl. Instead they put it on its stand, and it gives light to everyone in the house. In the same way, let your light shine before others, that they may see your good deeds and glorify your Father in heaven.”
Subscribe to The Kingston Report
The Kingston Report is an evidence-based analysis of COVID-19 causes, guidelines, treatments, ‘vaccines’, and market forecast presenting government documents not found on any other platform.